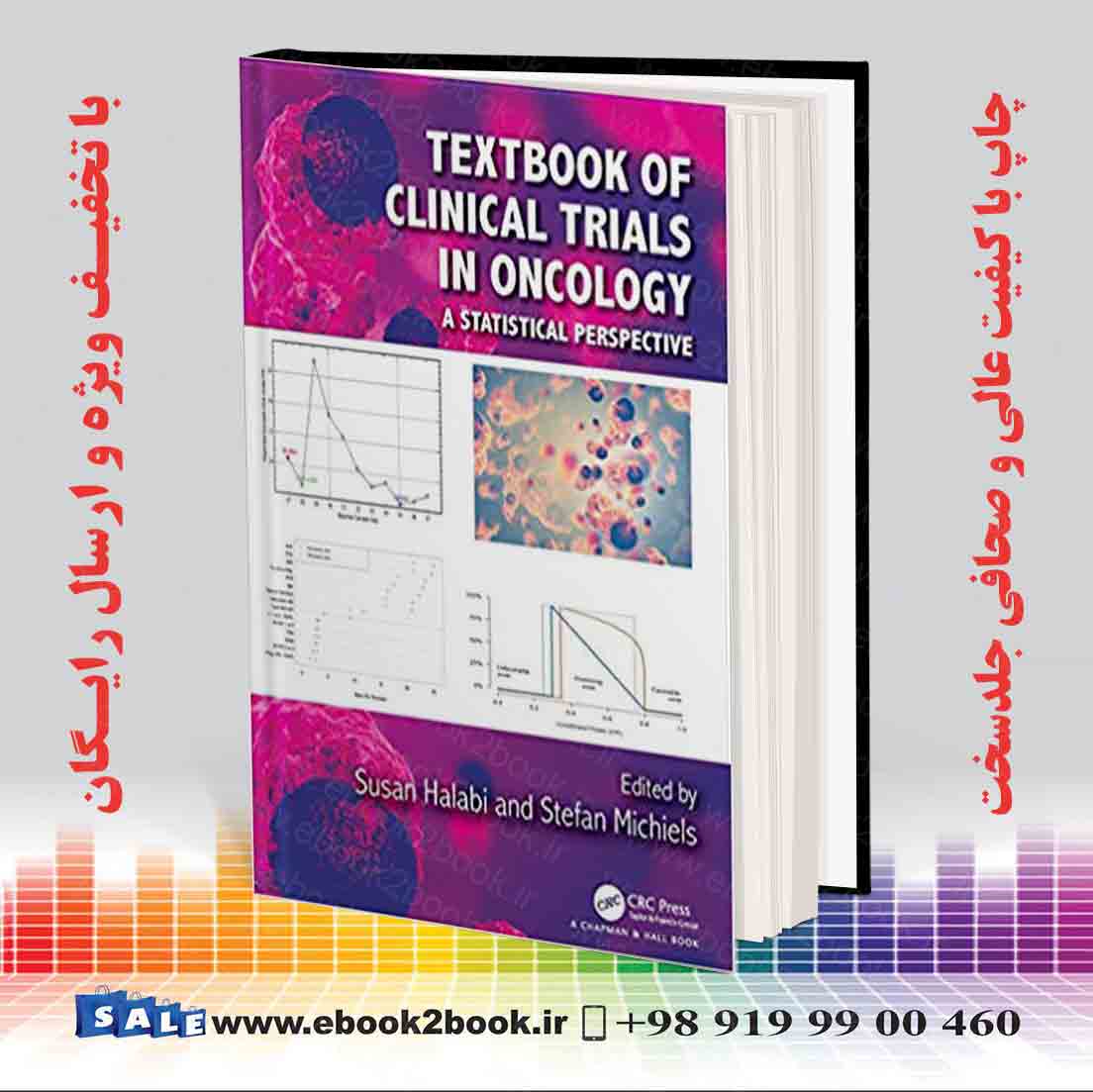
- Hardcover: 644 pages
- Publisher: Chapman and Hall/CRC; 1 edition (May 16, 2019)
- Language: English
- ISBN-10: 1138083771
- ISBN-13: 978-۱۱۳۸۰۸۳۷۷۶
- Product Dimensions: 7 x 1.4 x 10 inches
- # Oncology (Books)
- فروشگاه کتاب زبان اصلیجدید
- کتاب پزشکی
- کتاب های علوم پایه پزشکی
- کتاب های جراحی
- کتاب های پزشکی طب داخلی
- کتاب های آسیب شناسی
- کتاب های رادیولوژی
- کتاب های چشم پزشکی
- کتاب های تشخیص پزشکی
- کتاب های کودکان و نوزادان
- کتاب های پزشکی زنان و زایمان
- کتاب های پرستاری
- کتاب های مغز و اعصاب
- کتاب های بیهوشی و مراقبت های ویژه
- کتاب های پزشکی ورزشی
- کتاب های طب اورژانس
- کتاب های علوم دارویی و سم شناسی
- کتاب های طب جایگزین
- کتاب های مهندسی پزشکی
- کتاب های طب فیزیکی و توانبخشی
- کتاب های فناوری اطلاعات سلامت
- کتاب های پیراپزشکی
- کتاب های روانپزشکی و روانشناسی
- کتاب دندانپزشکی
- آزمون های بین المللی
- کتاب دانشگاهی
- کتاب های دامپزشکی زبان اصلی
- کتاب های زیست شناسی
- کتاب های مالی و بازار سرمایه
- کتاب های تجارت و اقتصاد
- کتاب های مدیریت زبان اصلی
- کتاب های ریاضیات و آمار
- کتاب های فیزیک زبان اصلی
- کتاب های مکانیک زبان اصلی
- کتاب های شیمی زبان اصلی
- کتاب های برق-الکترونیک
- کتاب های زمین شناسی
- کتاب های گیاه شناسی
- کتاب های حقوق
- کتاب های کیهان شناسی
- کتاب های بهداشت، ایمنی و محیط زیست
- کتاب های عمران، معماری، شهرسازی
- کتاب کامپیوتر
- کتاب خلبانی
- منوی کاربری
- فروشگاه کتاب زبان اصلی
- کتاب پزشکی
- کتاب های علوم پایه پزشکی
- کتاب های جراحی
- کتاب های پزشکی طب داخلی
- کتاب های آسیب شناسی
- کتاب های رادیولوژی
- کتاب های چشم پزشکی
- کتاب های تشخیص پزشکی
- کتاب های کودکان و نوزادان
- کتاب های پزشکی زنان و زایمان
- کتاب های پرستاری
- کتاب های مغز و اعصاب
- کتاب های بیهوشی و مراقبت های ویژه
- کتاب های پزشکی ورزشی
- کتاب های طب اورژانس
- کتاب های علوم دارویی و سم شناسی
- کتاب های طب جایگزین
- کتاب های مهندسی پزشکی
- کتاب های طب فیزیکی و توانبخشی
- کتاب های فناوری اطلاعات سلامت
- کتاب های پیراپزشکی
- کتاب های روانپزشکی و روانشناسی
- کتاب دندانپزشکی
- آزمون های بین المللی
- کتاب دانشگاهی
- کتاب های دامپزشکی زبان اصلی
- کتاب های زیست شناسی
- کتاب های مالی و بازار سرمایه
- کتاب های تجارت و اقتصاد
- کتاب های مدیریت زبان اصلی
- کتاب های ریاضیات و آمار
- کتاب های فیزیک زبان اصلی
- کتاب های مکانیک زبان اصلی
- کتاب های شیمی زبان اصلی
- کتاب های برق-الکترونیک
- کتاب های زمین شناسی
- کتاب های گیاه شناسی
- کتاب های حقوق
- کتاب های کیهان شناسی
- کتاب های بهداشت، ایمنی و محیط زیست
- کتاب های عمران، معماری، شهرسازی
- کتاب کامپیوتر
- کتاب خلبانی
- منوی کاربری
پشتیبان 99004600919
پشتیبانی

نقد و بررسیها0
هنوز بررسیای ثبت نشده است.